BACKGROUND: Measurable residual disease (MRD) before and/or shortly after allogeneic hematopoietic cell transplantation (HCT) is an independent indicator of high post-HCT relapse risk in acute myeloid leukemia (AML). However, many relapses, particularly among patients with MRD, occur within the first 3 months after allografting, and the role of MRD testing in the identification of patients at risk of relapses occurring at later times is unknown.
METHODS: To address this uncertainty, we studied all adults ≥18 years with AML or MDS/AML based on 2022 International Consensus Classification criteria who received a first allograft while in first or second morphologic remission at a single institution between 4/2006 and 3/2023 and underwent MRD testing via multiparameter flow cytometry during the pre-HCT evaluation. In line with the performance characteristics of the assay, any detectable MRD was considered positive.
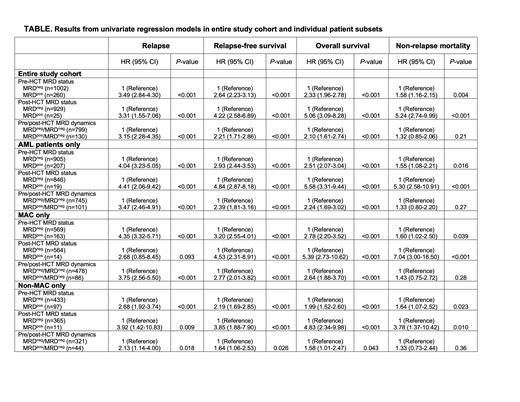
RESULTS: One thousand two hundred and sixty-two patients with AML (n=1,112 [88%]) or MDS/AML (n=150 [12%])underwent allograft following myeloablative (n=732 [58%]) or non-myeloablative (n=530 [42%]) conditioning regimens. Of these, 260 (21%) tested positive for MRD during the pre-HCT evaluation. With a median follow-up of 60.6 (range: 1.9-196.3) months after HCT among survivors, there were 589 deaths, 381 relapses, and 259 NRM events contributing to the probability estimates for relapse, overall survival (OS), relapse-free survival (RFS), and non-relapse mortality (NRM) in this cohort. 239/1,262 patients (19%) experienced disease recurrence (n=181 [14%]) and/or death (n=89 [7%]) within the first 100 days after allografting. Relapse ( P<0.001) and death ( P=0.002) but not NRM ( P=0.29) before day +100 were more common among patients with pre-HCT MRD. Of patients alive without relapse at day +100, 93% (954 of 1,023) underwent bone marrow restaging studies between day 70 and 100 post-HCT. Among these 954 patients, 146 (15%) had MRD before HCT, whereas only 25 (3%) had MRD at day +70-100 testing. The latter had a significantly higher 3-year relapse risk (37% vs. 18%; P=0.002), and worse RFS (17% vs. 68%; P<0.001) OS (16% vs. 72%; P<0.001) and NRM (46% vs. 14%; P<0.001) relative to the 929 patients without MRD; for results of univariate regression models, see Table 1. Importantly, however, while 130 of the 146 patients (89%) with MRD before HCT tested negative for MRD at day +70-100, their outcomes were substantially worse compared to patients who tested negative for MRD before HCT and at day +70-100, with 3-year relapse risk of 38% vs. 15% ( P<0.001), 3-year RFS of 47% vs. 71% ( P<0.001), and 3-year OS of 53% vs. 75% ( P<0.001), whereas 3-year NRM estimates were similar (14% vs. 14% ( P=0.21). Qualitatively similar results were obtained when the cohort was restricted to patients with AML, and also when comparing patients undergoing myeloablative conditioning with those undergoing non-myeloablative conditioning, with a statistically significantly higher relapse risk and lower RFS and OS but not NRM for patients with MRD before HCT but no MRD at day +70-100 compared to those who tested negative for MRD before HCT and at day +70-100 (Table 1).
CONCLUSION: For AML and MDS/AML patients alive without relapse at day +100 after allografting, MRD testing at day +70-100 post-HCT identifies patients at increased risk of relapse and worse survival, but only a small number of patients will test positive at this post-HCT timepoint. While a high proportion of patients with MRD before HCT will test negative for MRD at day +70-100, their outcomes are substantially inferior to patients without MRD both before and at day +70-100 after HCT. These data suggest that all patients with pre-HCT MRD should be considered for pre-emptive therapeutic strategies, ideally in the setting of well-controlled clinical trials, given their high risk of disease recurrence regardless of the post-HCT MRD information.
Disclosures
Milano:ExCellThera Inc.: Research Funding. Sandmaier:Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Walter:Amgen, Aptevo, Celgene, Janssen, Jazz, MacroGenics, Pfizer: Research Funding; ImmunoGen, Jura: Consultancy, Research Funding; Abbvie, Adicet, Amphivena, BerGenBio, Bristol Myers Squibb, GlaxoSmithKline, Orum: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal